Home » CBSE Guide » Class 10 » Science » Ch-1 In-Text Questions and Solutions
Chapter 1 - Chemical Reactions and Equations
In-Text Questions and Solutions
Page 3 of 3
19) Why are decomposition reactions called the opposite of combination reaction? Write equation for these reactions.
Ans) In a combination reaction, two or more substances combine to form a single substance along with the release of large amounts of heat whereas in a decomposition reaction, a single compound breaks into two or more simpler compounds and for this reaction to take place energy is required either in the form of heat, light or electricity.
20) Write one equation each for decomposition reactions where energy is supplied in the form of heat, light or electricity.
Ans) Thermal decomposition- When a decomposition reaction is carried out by heating, it is called ‘thermal decomposition’.
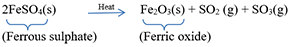
Example: Ferrous sulphate crystals (FeSO4, 7H2O) lose water when heated and the colour of the crystals changes. It then decomposes to ferric oxide (Fe2O3), sulphur dioxide (SO2) and sulphur trioxide (SO3).
Photolytic decomposition- When a decomposition reaction is carried out by using light energy, it is called photolytic decomposition White silver chloride turns grey in sunlight. This is due to the decomposition of silver chloride into silver and chlorine by light.

Electrolytic decomposition- When a decomposition reaction is carried out by using electric current, it is called electrolytic decomposition.
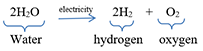
Example: When electric current is passed through acidified water, it decomposes to give hydrogen gas and oxygen gas. This decomposition reaction takes place by the action of electricity. It is called electrolysis of water
21) What is the difference between displacement and double displacement reactions? Write equations for these reactions. Ans) Displacement reaction occurs when a more reactive element displaces a less reactive element from its compound.
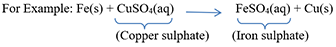
In this reaction, iron has displaced or removed another element, copper, from copper sulphate solution.
In a double displacement reaction, two atoms or a group of atoms(ions) are exchanged between the reactants to form new compounds.
For Example: When barium chloride solution is added to sodium sulphate solution, then a white precipitate of barium sulphate is formed along with sodium chloride solution.
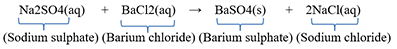
22) In the refining of silver, the recovery of silver from silver nitrate solution involved displacement by copper metal. Write down the reaction involved.
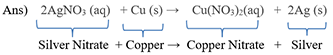
23) What do you mean by a precipitation reaction? Explain by giving examples.
Ans) Those reactions in which reactants react to form an insoluble solid called precipitate that separates from the solution is called a precipitation reaction.
Example:
(i)Precipitate of barium sulphate is formed along with sodium chloride solution.
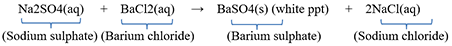
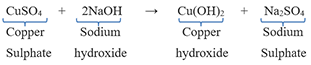
(ii) Copper sulfate solution reacting with sodium hydroxide solution. Copper solutions form a blue precipitate with sodium hydroxide.
(blue solution + colourless solution → blue precipitate + colourless solution)
24)Explain the following in terms of gain or loss of oxygen with two examples each.
(a) Oxidation (b) Reduction
Ans) (a) Oxidation is a chemical process in which a substance gains oxygen or loses hydrogen.
Example:
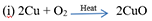
Copper gains oxygen and gets oxidised in copper(II) oxide.
(ii) H2S + Br2 → 2HBr + S
Sulphur loses hydrogen and gets oxidized.
(b) Reduction is a chemical process in which a substance gains hydrogen or loses oxygen.
Examples:
(i) CuO + H2 → Cu + H2O
Copper loses oxygen and gets reduced
(ii)H2S + Cl2 → 2 HCl + S
Sulphur loses hydrogen and gets reduced.
25) A shiny brown coloured element ‘X’ on heating in air becomes black in colour. Name the element ‘X’ and the black coloured compound formed.
Ans) ‘X’ is copper (Cu) metal. On heating in the presence of air, it gets oxidised to form a black-coloured compound namely Copper oxide(CuO). The equation of the reaction involved on heating copper is given below.

26) Why do we apply paint on iron articles?
Ans) We apply paint on iron articles because it prevents them from rusting. When the surface of iron is painted, its surface does not come into contact with oxygen and moisture. Hence, rusting is prevented.
27) Oil and fat containing food items are flushed with nitrogen. Why?
Ans) Oil and fat containing food items flushed with nitrogen because oil and fat become rancid on oxidation and their smell and taste change. By flushing the oil and fat containing food items with nitrogen, the nitrogen acts as an antioxidant and it prevent them from being oxidised.
28) Explain the following terms with one example each. (a) Corrosion (b) Rancidity
Ans) (a) When a metal is attacked by substances around it such as moisture, acids, etc., it is said to corrode and this process is called corrosion. The black coating on silver and the green coating on copper are other examples of corrosion
(b) The taste and odour of food materials containing fat and oil changes when they are left exposed to air for long time. This is called rancidity. It is caused due to oxidation of fat and oil present in food material. For example, butter becomes sour in taste and gives a foul smell when kept at room temperature for a longer time due to oxidation.
« Back to Menu | Page 1 | Page 2 | Page 3

