Home » CBSE Guide » Class 10 » Science » Ch-1 Exemplar Questions and Solutions
Chapter 1 - Chemical Reactions and Equations
Exemplar Questions and Solutions
MCQs
Page 3 of 3
13. In the double displacement reaction between aqueous potassium iodide and aqueous lead nitrate, a yellow precipitate of lead iodide is formed. While performing the activity if lead nitrate is not available, which of the following can be used in place of lead nitrate?
(a) Lead sulphate (insoluble)
(b) Lead acetate
(c) Ammonium nitrate
(d) Potassium sulphate
Ans) (b) Lead acetate
14. Which of the following gases can be used for storage of fresh sample of oil for a long time?
(a) Carbon dioxide or oxygen
(b) Nitrogen or oxygen
(c) Carbon dioxide or helium
(d) Helium or nitrogen
Ans) (d) Helium or nitrogen
15. The following reaction is used for the preparation of oxygen gas in the laboratory
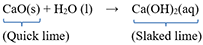
Which of the following statement(s) is (are) correct about the reaction?
(a) It is a decomposition reaction and endothermic in nature
(b) It is a combination reaction
(c) It is a decomposition reaction and accompanied by release of heat
(d) It is a photochemical decomposition reaction and exothermic in nature
Ans) (a) It is a decomposition reaction and endothermic in nature
16. Which one of the following processes involve chemical reactions?
(a) Storing of oxygen gas under pressure in a gas cylinder
(b) Liquefaction of air
(c) Keeping petrol in a china dish in the open
(d) Heating copper wire in presence of air at high temperature
Ans) (d) Heating copper wire in presence of air at high temperature.
17)In which of the following chemical equations, the abbreviations represent the correct states of the reactants and products involved at reaction temperature?
a) 2H2 (l) + O2 (l) → 2H2O (g)
b) 2H2 (g) + O2 (l) → 2H2O (g)
c) 2H2 (g) + O2 (g) → 2H2O (l)
d) 2H2 (g) + O2 (g) → 2H2O (g)
Ans) (c) 2H2 (g) + O2 (g) → 2H2O (l)
18)Which of the following are combination reactions?
i) 2KClO3 → 2KCl + 3O2
ii) MgO + H2O → Mg(OH)2
iii) 4Al + 3O2 → 2Al2O3
iv) Zn + FeSO4 → ZnSO4 + Fe
(a) (i) and (iii) (b) (iii) and (iv)
(c) (ii) and (iv) (d) (ii) and (iii)
Ans) (d) (ii) and (iii)
« Back to Menu | Page 1 | Page 2 | Page 3

